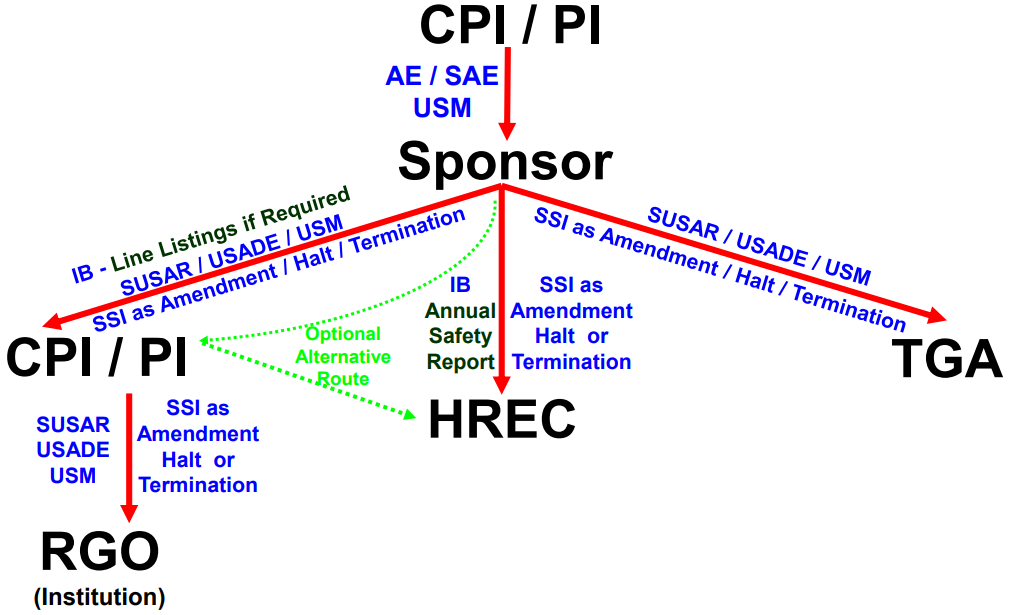
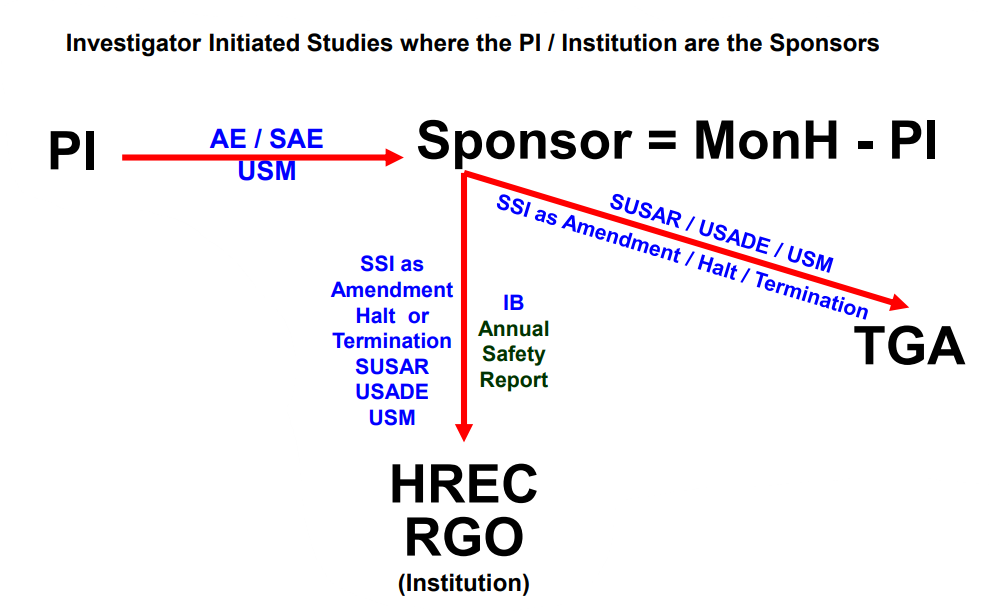
Research Ethics and Governance Safety Reporting procedure has recently been updated!
As per the updated Safety Reporting procedure, SUSARs are no longer accepted via RiskMan. Please report SUSARs via the Site Notification Form on Ethical Review Manager (ERM).
(Updated November 2025)