- Monash Childrens Hospital
- 7 min read

‘I just find it mind-blowing – amazing – that we found out everything before Mitchell was born and then were able to save him!’ Mitchell’s dad, Adam Hooper.
The finding afforded doctors time to put in place a life-saving plan for his birth and led to the use of an Australian-first therapy at Monash Children’s Hospital.

‘Mitchell’s case demonstrates the power of genomic sequencing. It allows us to be proactive in the care we provide, and that’s a game-changer,’ said Dr Andrew Fennell, Interim Director of General Genetics at Monash Health.
Lifesaving diagnosis
Lakes Entrance couple Melissa Nord and Adam Hooper were excitedly preparing for the birth of their second child when a routine ultrasound at 27 weeks gestation picked up a potential abnormality in his heart.
‘There were some areas where it should have been smooth and it was rippled,’ said mum Melissa.
The family was referred to specialists at Melbourne’s Monash Medical Centre, who suggested a cutting-edge prenatal genomic test to determine if Mitchell had a genetic heart condition.

Trio exome sequencing reads and compares the DNA of a child and their two biological parents to identify the cause of a genetic condition.
A sample of Mitchell’s DNA was obtained via amniocentesis at 31 weeks gestation.
The test found the cause of his heart abnormality – a genetic change inherited from his father, which could be managed through surveillance.
Unexpectedly, the comprehensive genomic test also predicted Mitchell would be born with the rare blood disorder, congenital thrombotic thrombocytopenic purpura (cTTP).
cTTP affects approximately one in one million people.
It is caused by a deficiency of the ADAMTS13 enzyme, which is vital to prevent excessive blood clotting.
‘Without intervention immediately after birth, babies can die from the complications of TTP as countless small blood clots form and cause damage to numerous organs, including the brain, heart and lungs,’ said Dr Fennell.
‘As parents it was very concerning, very scary,’ shared Melissa Nord.
‘It’s so rare, we didn’t really understand too much at all at the start,’ said Adam.
It is unusual for genomic testing to find something incidentally in the prenatal space.
‘Talking with the genetic pathologist in the genomics laboratory based at NSW Health Pathology we figured out the genetic changes could cause a major problem. But happily, there was an effective treatment we could administer,’ said Dr Fennell.
Preparations for delivery
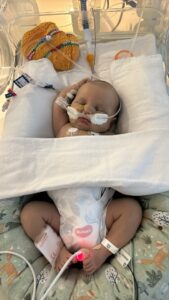
Preparations were made for Mitchell to be born at a hospital offering high-level care for complex pregnancies, Monash Medical Centre in Clayton.
He was delivered at 38 weeks gestation and administered fresh frozen plasma (FFP) containing the enzyme ADAMTS13 within an hour of being born. Precautions were in place in case low platelet levels caused bleeding.
‘Because cTTP is such a rare condition, and the features overlap with those of conditions which are much more common, the diagnosis may be missed in the neonatal period. Knowing that Mitchell was at risk of cTTP before he was born had a huge impact on his management,’ said Monash Health paediatric haematologist, Dr Luisa Clucas.
Mitchell was regularly administered fresh frozen plasma until he was 12 days old.
‘By the time he arrived, I felt very confident that they were going to save him, because we were very well prepared for what was to come,’ said Melissa.
‘We knew there was a high chance he could be born with the disease present, so we knew the medical team needed to have fresh plasma ready to save his life.’
‘We knew we would be in hospital for a long time, so we could prepare to have family there for our little girl and organise our paint shop business,’ said Adam.
An Australian first therapy

Infusions of fresh frozen plasma are the standard treatment for cTTP in Australia and prevent uncontrollable blood clots. But, the volume required is large relative to the size of a newborn.
‘Babies are tiny, so giving them the large amount of fresh frozen plasma required to replenish their ADAMTS13 enzyme level can lead to problems with fluid overload,’ said Dr Fennell.
As good fortune would have it, one week before Mitchell was born an advanced therapy administered in smaller volume, recombinant ADAMTS13, was approved by the Food and Drugs Administration in the United States.
The therapy contained the enzyme Mitchell needed without the other components that made fresh frozen plasma bulky.
Dr Clucas knew about trials of the medication and of a single case of a newborn being treated with it.
She approached the pharmaceutical company, Takeda, securing Mitchell urgent compassionate access.
‘It was exciting to be able to offer this treatment to Mitchell’s parents,’ said Dr Clucas.
The drug was not available locally having yet to be approved for use in Australia.
Australia’s Therapeutic Good Administration granted clinicians special dispensation to administer recombinant ADAMTS13 to Mitchell.
It was imported and administered to Mitch when he was 15 days old. He continues to be given it weekly.
‘If it wasn’t for Luisa reading an article about this drug being released in America, we wouldn’t have had it for Mitchell,’ said Adam.
‘Without that knowledge and everything that the whole team did, Mitch potentially wouldn’t be here!’
Ongoing care

Mitchell turned 2 this month.
His parents say he has become an energetic, cheeky little boy, much-loved by his 5-year-old sister, Lilah.
Once a week, Mitchell has an infusion through a port in his chest at Sale Regional Hospital.
‘We have our specialist at Monash Children’s Hospital, Luisa, on a Zoom call, and that means we don’t have to drive a 10-hour round-trip to Clayton every single week,’ said Melissa.
Once a month, the family travels from Lakes Entrance to Monash Children’s Hospital for the infusion and a physical checkup.
‘Mitchell was very sick when he was born. He still requires regular treatment, but seeing him as a healthy, happy toddler is amazing,’ said Dr Clucas.
Heart health

Genetic testing has provided another upside for the family.
It was revealed that Adam had a variation in his genes – Filamin C gene.
‘It just means as we get older that we’ve got to watch our hearts and make sure to have a few more checks than normal,’ shared Adam.
‘It’s a gene change that can be passed on. So, we watch both kids’ hearts very closely.’
Prenatal genetic testing
Monash Health did not do prenatal genomic sequencing before 2018.
‘It’s really only existed as a clinical test worldwide since 2017/2018 and even at that stage, we considered it research in prenatal medicine, because we didn’t have good evidence for the likelihood of finding something, when to appropriately use it, or how to safely use it. And so, it’s really exploded over the past few years,’ said Dr Fennell.
Mitchell’s prenatal genomic sequencing was funded through the Australian Government Medical Research Future Fund PreGen program led by Prof Tony Roscioli of Neuroscience Research Australia (NeuRA) and NSW Health Pathology. The study is looking at how best to care for families who undergo genomic testing in pregnancy and assessing the case for a Medicare item number for prenatal genomic testing.

Monash Health projects to carry out genomic sequencing of 70 to 80 fetuses this year – a combination of prenatal tests and tests carried out on fetuses who have passed away for unexplained reasons or with anomalies.
‘Two years ago, we completed genomic sequencing for 12 families in these circumstances. So, it’s a massive increase in our service provision.’
‘Researchers discover a new gene linked to a disease once or twice a week. Every research finding is fed almost immediately into genetic testing, making it better and better every week.’
Melissa and Adam received genetic counselling as part of the family’s care.
‘I think they felt confident going forward and reassured.’
‘It was a complicated case with two different genetic conditions, but with a positive outcome due to precision therapy that is increasingly becoming available in genetics. Almost no gene-targeted therapies existed a decade ago, but major progress in research means these are rapidly coming on-stream in the clinic and genetic testing is opening the door.’


